Heat capacity: definitions
Definition
The heat capacity of a material is the heat quantity necessary to raise its temperature by one degree.
The mathematical relationship expressing this definition has the form:
ΔQ = Cp . ΔT
where:
- ΔQ is the heat quantity (J)
- ΔT is the temperature variation (in degrees)
- Cp is the heat capacity at constant pressure (J/degree)
The heat capacity of a body is a quantity that offers the possibility to evaluate the amount of heat exchanged by this body during a process characterized by a temperature variation.
Specifications
The heat capacity Cp is an extensive quantity.
Thus in practice, the following quantities will be used:
-
specific heat capacity1:
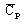
with
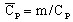 (J/kg/degree)
(J/kg/degree) -
volumetric heat capacity2:
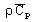
with
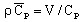 (J/m3/degree)
(J/m3/degree)
Dependence on temperature
The heat capacity can depend on the temperature: Cp=f(T). It increases as the temperature increase.
The variation of the heat capacities with temperature can provide important information about the crystalline microstructure of the material (see § Phase transition ).